by Ana Wang in collaboration with Angela Mitchell and Olivia Mullins
Vaccines prevent millions of illnesses and save incalculable numbers of lives.
There are a lot of stories, facts, and fictions circulating in the news and on social media about the COVID-19 vaccines. For those who are not scientists or medical professionals (or even sometimes those who are), it is difficult to distinguish between what is real and what is made up, especially when these stories speak to our deepest fears, like our safety and well-being.
For the last year, we have been encouraged to wear masks over our noses and mouths, wash our hands, maintain social distancing, and avoid large gatherings. Now that we have vaccines available, we must do our part and get vaccinated. We have put together an FAQ-style blurb to help you understand and feel comfortable (even enthusiastic!) about you, your family, and your friends getting the COVID-19 vaccine.
How do vaccines work?
Vaccines work by ramping up your immune system so your body can fight off pathogens that it encounters. How? Most vaccines contain inactivated pathogens or pieces of a pathogen – like a virus or bacterium. These weakened versions do not hurt you. They do, however, prompt your immune system to build up its defense so that if/when you are exposed to that full virus, your body will recognize and destroy it before it can get you sick.
(The COVID-19 vaccine uses a slightly different approach, which is described below!)
Did you know? Vaccines rely on our body’s natural immune processes.
Germs are everywhere - on all surfaces, in the air, even in and on your own body. As you are exposed to different environments, animals, and people, your immune system learns how to fight off disease-causing pathogens. Every time you get sick and recover, your immune system has learned a new skill – how to fight off a new pathogen. In this way, when you encounter that same pathogen again, you either do not get sick or you experience less severe symptoms because your immune system knows how to destroy it.
Why can’t we simply rely on our body’s immune system?
Despite our body’s abilities to fight off diseases, some viral or bacterial infections can cause severe illness, life-long health issues, or death. For many of these diseases, scientists have developed vaccines to “educate” our immune systems ahead of time. This reduces or eliminates the risk being severely ill or dying if you are exposed to the pathogen.
How exactly do vaccines work?
The majority of vaccines are made to protect against viruses. These vaccines usually contain either an inactivated (dead) virus, or they contain a protein that is on the surface of a virus particle (but the COVID-19 vaccine is different - see below!). You are never given the intact virus, so the vaccine is essentially tricking your immune system into thinking that you are actually infected.
The dead virus or small virus particle cannot infect you, but it does have a critical molecule called an antigen on it. When your body encounters a new antigen, it creates proteins called antibodies. Antibodies are a key component to your immune defense. Antibodies are specific and only recognize that particular antigen. Every time your immune system encounters a new antigen, it will create a new antibody. In this way, every antibody has its one, specific antigen match.
Once these new, unique antibodies are created, they stick around for years, decades, or a lifetime! The antibodies circulate around your body in your blood, on guard and ready to bind their matched antigen. If the antibody finds the antigen, it will trigger your immune system to attack and remove the pathogen. In this way, you are protected from getting sick.
Without the vaccine, your immune system could take weeks to respond to the new infection, and by then, it’s often too late.
If vaccines don’t have an active virus, why do they make me feel sick?
Have you ever gotten a flu vaccine and felt a little bit sick the next day? The fever and muscle aches that you feel are your immune system working! In fact, some symptoms when you are naturally infected are due to your immune system’s attempts to kill the pathogen.
The effects from vaccination are not the same as actually being infected and getting the full illness; that is why these effects do not last very long and are generally less severe than the actual illness.
After receiving the COVID-19 vaccine, you may have experienced symptoms yourself or heard of other people who felt feverish or achy for a day or two after their vaccine (usually after their second dose). However, this is no reason to be afraid of getting the vaccine. On the contrary – the symptoms experienced immediately after receiving the vaccine indicate that your immune system is building up defenses, and the vaccine is doing its job! Some people may never experience these symptoms after vaccination, and that is okay too. Everyone’s immune system will respond in its own way, and some people simply do not have side effects (but they are still protected just as well as those who do experience side effects).
Vaccination is like an old Western movie.
Vaccination is like an old Western movie when the town has “Wanted” posters everywhere. The citizens of the town are like your immune system, the criminal on the poster is like the virus, and the poster is like the vaccine. Once the citizens see the poster, they know to look out for the criminal. Vaccines are given to you so that if you are exposed to that full virus in the future, your body will recognize and destroy it before you get sick.
More information about how vaccines work can be found in the reference list below.
What are the risks of vaccines?
As with any vaccine or treatment, there may be allergic reactions in some people. Although extremely rare, the CDC suggests that people be monitored for 15 minutes after each vaccination, and for people with severe records of allergies, they recommend monitoring for 30 minutes.
This precaution should not deter you from getting the vaccine, though, because only 2-5 allergic cases out of one million have occurred (0.0002% - 0.0005%). As a comparison, ~1%, or 1 out of 100 people, are allergic to peanuts. Typically, cases of vaccine allergies have been in people with histories of severe allergies and anaphylaxis. If you fall into this category, be sure to notify the vaccine provider ahead of time, and they will inform you of how to best proceed.
Some people worry about long term effects of vaccines but as Dr. Fauci explains “virtually all long-term adverse effects of a vaccine occur between 15 and 30 days after you get the dose, 45 days at the most.” In contrast, many people have “long-COVID” with symptoms continuing after months, even over a year and evidence suggest it will cause long-term health effects in many people. Other diseases cause long-term effects as well.
What is an mRNA vaccine?
We established that vaccines commonly contain a viral protein, and this protein is the antigen that is recognized by your immune system to attack and clear out the virus, if you become exposed. However, the COVID-19 vaccines work a little differently. The first two vaccines approved and used in the USA are mRNA vaccines from Pfizer-BioNTech and Moderna. So, what is different about mRNA vaccines?
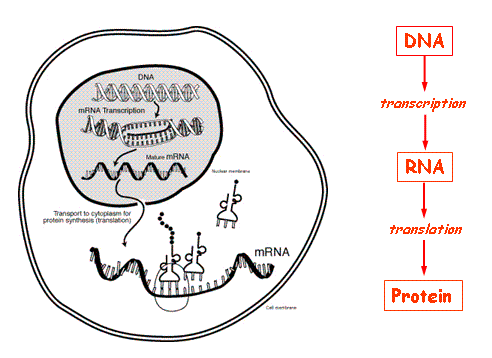
Brief background on DNA, RNA, and proteins
The principal concept in biology goes like this: You are born with DNA. That DNA is used as a template for creating another biological entity called messenger RNA (mRNA) in a process called “transcription.” mRNA is then read (or “translated”) into a protein, and proteins are the main players in all biological processes. This DNA → RNA → protein process is commonly known as The Central Dogma of Biology.
How does the mRNA vaccine work?
mRNA vaccines contain pieces of the virus, but instead of proteins from the virus, they contain a piece of mRNA from the virus. The mRNA is like a blueprint, or the instructions, for how to make a protein. So, rather than injecting the viral protein itself, mRNA vaccines are delivering the instructions for how to make the viral protein. Your body will take those instructions, make the viral protein, and then your immune system gets to work.
It is important to know that the mRNA in the vaccines do not have the instructions to make the full virus. Therefore, the vaccine can NEVER instruct your body to make the entire COVID-19 virus.
For the most common COVID-19 vaccines in the US (Pfizer-BioNTech and Moderna), the mRNA only has instructions for making what is called the spike protein. The spike protein is on the surface of the virus and binds to our cells, allowing the virus to enter the cells. Our bodies will create spike protein from the injected mRNA of the vaccine, so that in the future, if we are exposed to the SARS-CoV-2 virus, our immune system will recognize the spike protein and destroy the virus.
Have mRNA viruses been used before?
The COVID-19 vaccine is the first mRNA vaccine approved for use in humans. However, research focused on mRNA vaccines has been ongoing for decades. In fact, mRNA vaccines have been studied for many diseases (for example: Zika, Ebola, cancer, rabies, HIV), and there are several ongoing, promising vaccine clinical trials that may allow these vaccines to be approved for use.
mRNA is not harmful. Actually, RNA in general is very fragile, and a lot of mRNA vaccine research effort goes into keeping the mRNA from falling apart or from being destroyed before it can take effect. The mRNA vaccines do not affect or change your DNA in any way. After your cells read the “instructions” for how to make the protein, the mRNA is destroyed.
More info on mRNA vaccines can be found in the reference list below.
What are the advantages of using mRNA in vaccines instead of proteins?
mRNA vaccines are easier, cheaper, and quicker to make than protein vaccines. They can be generated in laboratories with materials that are easily and readily available. In contrast, some protein vaccines (such as the flu vaccine) are made in chicken eggs, which takes more time and money.
During a pandemic, time is of the essence. The longer it takes to generate millions of doses of a vaccine, the larger the amount of time the virus spreads and mutates. mRNA vaccines can be made and scaled up efficiently. This efficiency is also advantageous when dealing with viruses such as SARS-CoV-2 that mutate and evolve quickly. The shorter manufacturing time of mRNA vaccines means we can create and distribute new versions of a vaccine quickly to curb the spread of the disease caused by mutated viruses.
What are the scientific differences between the available vaccines?
The Pfizer-BioNTech and Moderna vaccines are mRNA vaccines (explained above). The Johnson & Johnson vaccine uses a different approach, known as a viral vector vaccine. It uses a different harmless virus, called an adenovirus, to carry the instructions for making the SARS-CoV-2 spike protein. The adenovirus does not cause an infection, but it does enter the cells and use the instructions to make the spike protein. From there, your immune system takes over as described in the previous sections.
In clinical trials, the Pfizer-BioNTech and Moderna vaccines showed 95% and 94.1% efficacy, respectively, after two doses. Johnson & Johnson’s vaccine is a bit different. It requires only one dose and has shown 72% protection against COVID-19 infections and 85% protection against severe disease in clinical trials in the USA. Johnson & Johnson is also testing the efficacy if two doses are used, but these results are not expected until May 2021, at the earliest.
More info on the current SARS-CoV-2 mRNA vaccines can be found in the reference list below.
What is herd immunity, and how does it work?
Herd immunity (a.k.a. population immunity) is what occurs when enough people in a population are immune to a disease that it provides indirect protection to those who are not immune. For COVID-19, it is estimated that we will achieve herd immunity when 70-75% of people are immune to the disease. If 80% of the population is immune, that means 4 of every 5 people will be protected. The higher the percentage of immunity, the larger the benefit to the population as a whole.
Many diseases, such as measles, mumps, chicken pox, and polio, are now very rare in the USA because vaccines helped establish herd immunity for each of these diseases. To be clear, with herd immunity, the spread of the disease is reduced in frequency, but it does not mean that the disease is eliminated. Likely, rare cases of the disease will still appear among the population’s most vulnerable members. However, with the vast majority of the population protected, we will have the space and resources in medical facilities to provide proper care for sick individuals.
How do we achieve herd immunity?
There are two ways to achieve herd immunity – people get sick and recover from the virus (bad) or they get vaccinated (good). The problem with the former option is that with diseases like COVID-19, millions of people die along the way to herd immunity.
There are many factors involved in reaching herd immunity. While we are getting vaccinated to reach that herd immunity threshold, we must maintain precautions to slow the spread of the virus. If society “re-opens” too soon, infection rates will increase again, prolonging the process and endangering more lives. Furthermore, the more people that are infected, the more chances the virus has to mutate, which increases the risk that our vaccine efforts are less effective or ineffective. If a virus takes over that has mutated away from the vaccine’s protection, new vaccines will need to be developed.
In the best-case scenario, people will get vaccinated as quickly as possible while maintaining social distancing, wearing masks, and following CDC guidelines, even if they are already vaccinated. We will have the best chance of going “back to normal” in the summer of 2021 if we are able to slow the spread. It requires everyone’s cooperation.
More info on herd immunity can be found in the reference list below.
Should you get vaccinated?
YES! Get vaccinated for yourself, for your loved ones, and for everyone else in the world. The more quickly we reach herd immunity, the more lives we will save and the more quickly we can travel and gather again safely!
Do I need to keep wearing a mask, washing my hands, and taking other precautions after I am fully vaccinated?
You should maintain all recommended CDC guidelines. At of the time of this writing, the CDC allows fully vaccinated individuals (two weeks after your second dose) to be outside without masks. Data suggests that transmission is greatly reduced – but not proven to be eliminated – when you are fully vaccinated. Vaccinated individuals can potentially be carriers of the virus; just because you do not get sick from it does not mean you cannot carry and transmit the virus to someone else. Extra care should be taken indoors and around high-risk people, even when vaccinated. And you should always wash your hands!
Importantly, it is not entirely known how effective these vaccines are for the emerging new strains (mutants) of SARS-CoV-2. Mutations can occur in the spike protein, which is the antigen that the vaccines are targeting. Many virus variants have emerged, and variants will continue to emerge as people continue to get infected and spread the virus around. Because of these variants, we may need to get additional booster shots of the current vaccines, or new COVID-19 vaccines may need to be developed in the future. Because we don’t know how the current vaccines will fair against any future mutants, we should continue implementing safety precautions.
In short, be safe and considerate. By continuing to take precautions, you are protecting yourself and others!
References:
“How do vaccines work?” World Health Organization. https://www.who.int/news-room/feature-stories/detail/how-do-vaccines-work. 14 April 2021.
mRNA Vaccines
“RNA vaccines: an introduction.” Foundation for Genomics and Population Health. https://www.phgfoundation.org/briefing/rna-vaccines. 14 April 2021.
Pardi, N., Hogan, M., Porter, F. et al. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov 17, 261–279 (2018).
Chahal JS, Kahn OF, Cooper CL, et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc Natl Acad Sci USA 13(29): E4133-42 (2016).
SARS-CoV-2 mRNA vaccine
“Understanding mRNA COVID-19 vaccines.” Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html. 14 April 2021.
“Comparing the COVID-19 Vaccines: How Are They Different?” Yale Medicine. https://www.yalemedicine.org/news/covid-19-vaccine-comparison. 14 April 2021
Herd Immunity
“What is Herd Immunity and How Can We Achieve It With COVID-19?” Johns Hopkins Bloomberg School of Public Health. https://www.jhsph.edu/covid-19/articles/achieving-herd-immunity-with-covid19.html. 14 April 2021.