When we think of scientists, thoughts of lab mice are close behind (à la Pinky and the Brain) but did you know that scientists use many OTHER organisms to study both basic and biomedical sciences? Just imagine: biomedical research using fruit flies could relate directly back to you, who, relative to a fruit fly, are much larger and seemingly more complex!
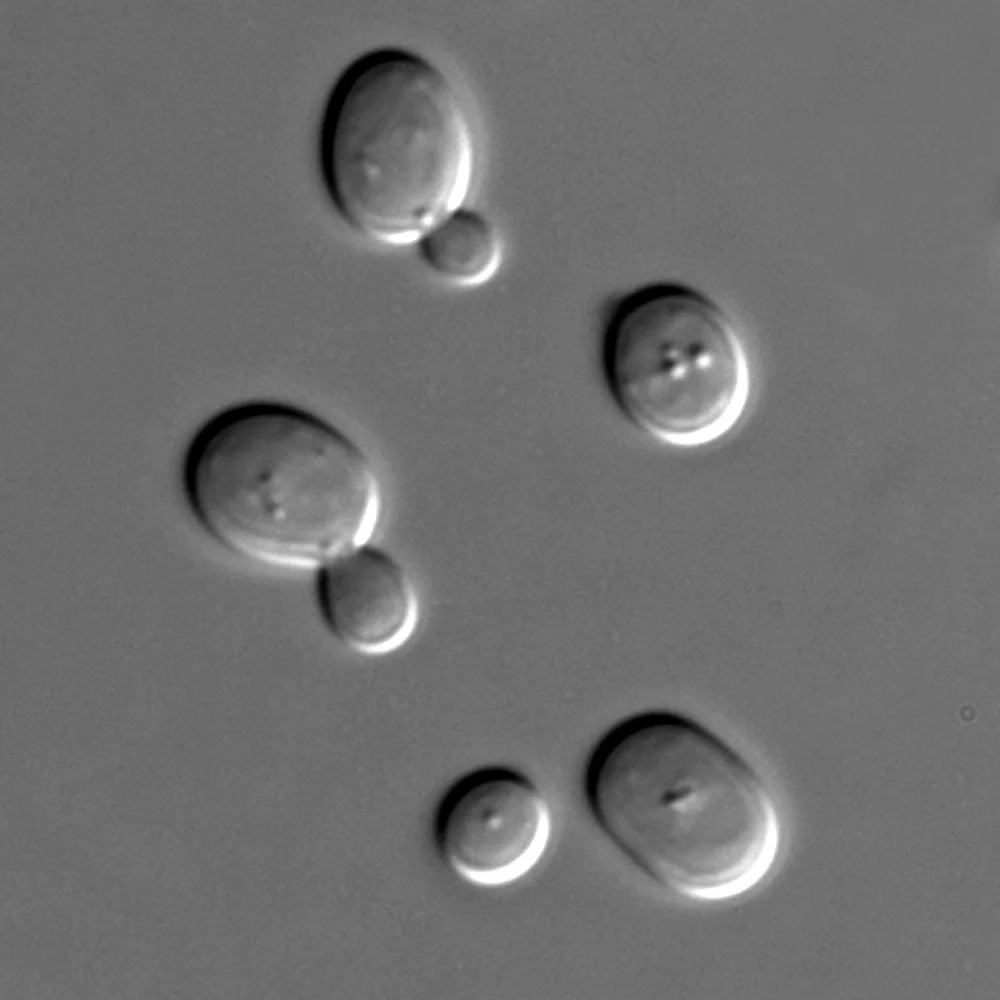
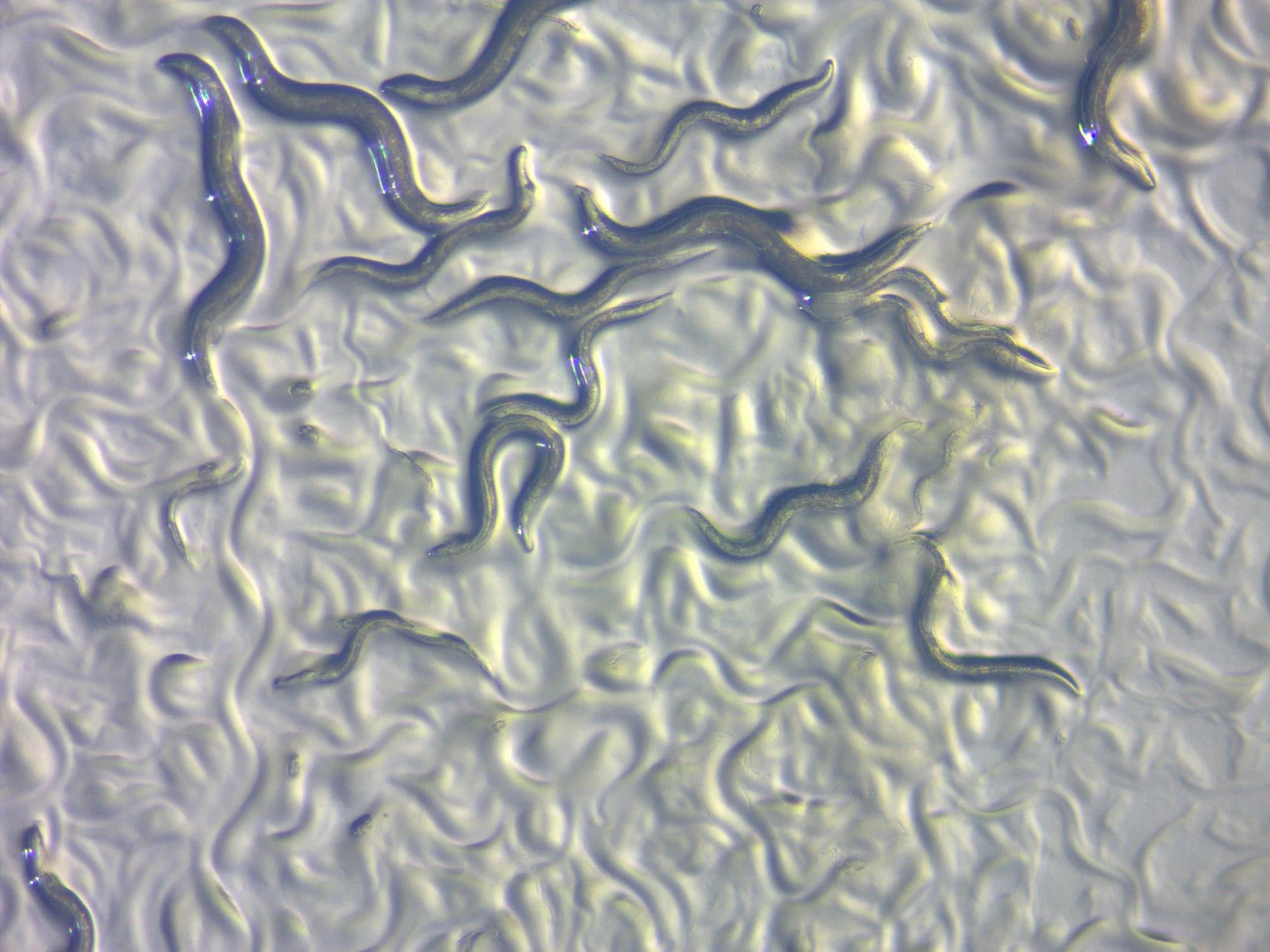
Model organisms are any non-human species that scientists use to understand complex biological processes. Good model organisms are easy to breed, have short life cycles and are often physically small, making them easy to maintain in laboratories and manipulate in experiments. Outside of rodents, common model organisms include, but are not limited to: fruit flies (Drosophila melanogaster), nematode worms (C.elegans), yeast (Saccharomyces cerevisia), zebrafish (Danio rerio), and sea urchins (Arbacia).
As expected, model organisms are great at being, well…models. So, it may be hard to imagine how they can be used to understand human health. However, believe it or not, these organisms are great at doing just that! Despite looking dramatically different from humans, many of the key genes in model organisms have gene counterparts (homologs) in humans and other large mammals. This is why fruit flies are useful to scientists study cleft palate and the mechanisms behind diabetes…though more about that later. First, it is important to understand that non-rodent organisms are a hallmark of scientific research and that their influence on scientific progress cannot be overlooked. Some of these species have even earned scientists Nobel Prizes.
Major biological advances via some unexpected organisms
To grow and develop, organisms need to make new cells from older cells. How does this process work? And how do organisms get rid of these old cells or cells that are “bad”? Using model organisms like yeast and sea urchins, scientists answered these fundamental questions (by discovering the mechanisms behind the cell cycle and apoptosis, respectively) and as a result, expanded our understanding of diseases like cancer at the same time. Yes, sea urchins, like the ones you see at the aquarium, can help us fight cancer!
In 2001, three scientists won the Nobel Prize for discovering key proteins important in a molecular process called the cell cycle using yeast and sea urchins. The cell cycle is the process by which cells make more cells. They do this through duplication and division of their genetic material and cell growth; it is a highly regulated process. Since cells are the basic building blocks of all living organisms, scientists were lucky to find the ideal model organisms to dissect this mechanism. The first model organism used, yeast, are single-celled, simple eukaryotic organisms whose genome can be easily manipulated, making them popular for genetic studies. They are also inexpensive and easy to grow in lab. Picture Erlenmeyer flasks full of yeast! Sea urchins, the other system used, have eggs that can be harvested and fertilized outside of the animal and, when initiated, divide synchronously, making cell division and development easy to visualize. Using these organisms, the Nobel Prize-winning research discovered several principle cell cycle proteins called cyclins and cyclin dependent kinases (CDKs).
Excitingly, these findings about the cell cycle not only developed our basic understanding of cells but set a foundation from which scientists could better understand disease. Cancer is characterized by a lack of cell cycle regulation. Tumors are the result of acquired genetic mutations and uncontrolled cell growth. Yeast and sea urchins, which appear so different from us, enabled scientists to understand fundamental biological processes yet also also provided mechanisms to target in cancer research and treatment. As startling as it may sound, this was not the only time model organisms helped scientists make landmark, prize-winning discoveries. It has occurred countless times throughout science history!
The following year, 2002, three more scientists won the Nobel Prize for their work developing “nematode worms” (C.elegans) as a model organism and using them to study a process scientists call “programmed cell death” or more technically speaking, apoptosis. This is like it sounds. Cells are programmed to self-destruct and this apoptosis is a necessary part of healthy development. Like the cell cycle, understanding apoptosis also improved our understanding of human health. One of the major advantages of C.elegans is that scientists can actually track each individual cell during a worm's development under a microscope. Utilizing the uniqueness of this organisms, scientists observed that the same 131 cells of the total 1090 cells in a developing worm consistently died, indicating that apoptosis was a natural and necessary process and this, in part, led to the identification of apoptosis genes. Apoptosis is evolutionary conserved in other organisms and defects in the process can cause human diseases. It is often triggered when healthy cells detect something wrong. In diseases like cancer, mutations in apoptosis genes allow unhealthy and dysfunctional cells to continue growing. Conversely, an increase in cell death of specific cell types contribute to some neurodegenerative diseases such as Alzheimer’s and Huntington’s Disease.
They are more like us (humans) than you would Think!
Hopefully it is now clear that non-rodent model organisms are crucial tools used by scientists to dissect basic biological processes, but don’t forget - these organisms can also more directly model human health. Just like with mice, there are a lot of genes in these other organisms that have homologs in humans, making them also great for modeling diseases and drug development.
For instance, in 1980, scientists discovered the hedgehog gene (hh) which is necessary for Drosophila (fruit fly) body patterning. Soon after, scientists found three similar genes in humans. One of these genes, sonic hedgehog (shh) – yes, it is named after the video game – is also important in early human development. Defects in human shh can result in cleft palate birth defects, which occur in 1 of every 1000 newborns. Scientists are therefore using flies to better understand the underlying mechanisms of cleft palate defects and using these studies to guide future research in larger mammals.
Now these same flies are also helping scientists understand the mechanisms behind diabetes. Despite being different from humans, insulin affects humans and flies similarly. In humans, insulin regulates the ability of cells to absorb glucose from the blood. In diabetes, this process is blocked. When scientists mutated several insulin-like genes in fruit flies, the insects developed diabetic symptoms and preliminary studies suggested that the cellular reason for their symptoms could be similar to those in humans. The hope is to use these fly models to create drugs and treatments that target these commonalities between flies and humans to ultimately combat diabetes.
Of course, as with any model, each system has its limitations, but the importance of using model organisms is clearly not a thing of the past. Studying model organisms and using the information we gain from them serve as the basis of many medical advances. Plus remember: some of the most seminal science discoveries that fill our textbooks were done in these species! Science is an abyss of unknown and model organisms have been, and will continue to be, a vital tool in discovery. Though these studies can stand alone, they also set a foundation for future studies in larger mammals (humans), saving scientists and society time, energy and money.
So the next time you see a fruit fly buzzing around your house, as annoying as it may seem, keep in mind that the species helped make countless scientific breakthroughs.
References:
- “What are model organisms?” Your genome. Public Engagement team and Wellcome Genome Campus, 03 March 2017. http://www.yourgenome.org/facts/what-are-model-organisms. 03 August 2017.
- "The Nobel Prize in Physiology or Medicine for 2001 - Press Release". Nobelprize.org. Nobel Media AB 2014, 08 October 2001. http://www.nobelprize.org/nobel_prizes/medicine/laureates/2001/press.html. 03 Aug 2017.
- "The Nobel Prize in Physiology or Medicine for 2002 - Press Release". Nobelprize.org. Nobel Media AB 2014, 07 October 2002. http://www.nobelprize.org/nobel_prizes/medicine/laureates/2002/press.html. 03 Aug 2017.
- Bedard, James. “Cleft Palate. Defects in Midline Facial Development.” modECODE. Genetics Society of America. http://modencode.sciencemag.org/drosophila/cleftPalate/. 03 August 2017.
- “ ‘Diabetic flies’ can speed up disease-fighting research”. College of Computer, Mathematical & Natural Sciences. University of Maryland, 06 November 2013. https://cmns.umd.edu/news-events/features/1465. 03 August 2017.
Picture Credits:
- Header picture: Enlarged c elegans - By Courtesy: National Human Genome Research Institute - From http://www.genome.gov/10000570, Public Domain, https://commons.wikimedia.org/w/index.php?curid=785840
- Sea urchin - By Lacen - Croatia, Public Domain, https://commons.wikimedia.org/w/index.php?curid=248760
- Saccharomyces cerevisiae - By Masur - Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=1069017
- Small male Drosophila melanogaster fly - By André Karwath aka Aka - Own work, CC BY-SA 2.5, https://commons.wikimedia.org/w/index.php?curid=227170
- C. elegans worms - By ZEISS Microscopy from Germany - C. elegans, model organism in life sciences, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=52989519