Beach in Oahu, Hawaii
With warm weather and summer vacation just around the corner, it is difficult to escape the draw of our oceans and beaches. Almost all of us, especially here in San Diego and other beachside cities, have stories to tell about our oceans, whether it is a fond memory from a family trip to the beach, a fishing voyage with friends, or a solo surf session by a pier at sunset. However, the oceans also tell us stories, and the narrative becoming clearer and more imminent is that of the declining health of our oceans.
A factory in China. The Industrial Revolution brought about a reliance on burning fossil fuels for energy, which pumps large amounts of carbon dioxide and pollutants into our environment.
Since the Industrial Revolution, the use of fossil fuel-powered machinery has emitted billions of tons of carbon dioxide along with other gases into our atmosphere. Today, it is estimated that one million tons of carbon dioxide are emitted every hour – that’s a faster rate than has existed on our planet in tens of millions of years. Our oceans and seas absorb up to one-third of these gas emissions. This helps all of us on land because these greenhouse gases are taken out of the atmosphere, slowing down climate change. But, still, this comes at a cost.
All solutions are acids or bases, and the acidity or basicity of a solution is defined by its pH value. A pH of 7 is neutral - the pH of pure water. Above pH 7 is basic, and below pH 7 is acidic. The surfaces of our oceans are healthiest when they are slightly basic with a pH of 8.2. Because of the large amounts of carbon dioxide entering our atmosphere, the pH of our oceans is decreasing, which is a process called ocean acidification.
A scientist in Svalbard, Norway studying the effects of climate change on the oceans' chemistry.
Because of ocean acidifications, our oceans are now at pH 8.1, and at the current rate, the ocean’s pH is predicted to drop about 0.5 pH units before the end of the century! At a glance, this may seem like a small or insignificant change, but it is enough to cause very serious problems in biological systems. For example, human blood is normally between a pH of 7.35 and 7.45. A drop in pH of 0.2-0.3 can cause seizures, comas, and death. You can imagine the large impact such a change can have on an ecosystem that takes up over 70% of our planet! About 250 million years ago, large levels of volcanic activity caused a similar level of ocean acidification, and this change contributed to the death of 90% of marine species.
So, how does ocean acidification occur? Carbon dioxide in our atmosphere dissolves in water to make a molecule called carbonic acid, so as our oceans continue to absorb carbon dioxide, the waters become increasingly acidic. In the past, basic molecules created by weathering rocks and sediments were enough to balance the carbonic acid and keep the oceans at their ideal 8.2 pH. However, rock erosion is not fast enough to keep up with acidification caused by an increasing excess of carbon dioxide released into our atmosphere.
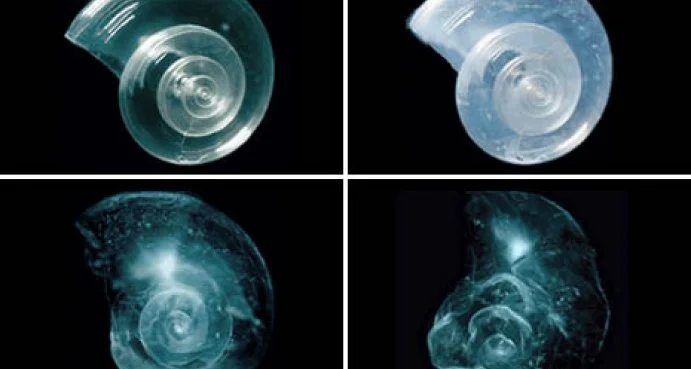
An experiment showing how acidic waters dissolve marine organisms' shells.
Such a rapid change in our oceans’ chemistry is not compatible with our marine organisms, which have evolved over millions of years in an ocean with a stable pH of 8.2. Ocean acidification affects marine organisms’ ability to communicate, reproduce, and grow. For example, at a healthy pH, about 10% of the carbon dioxide dissolved in the water exists as a molecule called carbonate. To make their shells, marine organisms like corals, clams, mussels, and oysters combine calcium with carbonate. Acidic waters have less carbonate, making it difficult for these animals to survive. Furthermore, acidic waters can chemically change the carbonate in the organisms’ shells to slowly dissolve them.
Acidic waters also lower the pH of the body fluids in all marine life, such as fishes, making it difficult for them to breathe and for their brains to function. It’s similar to if the air we breathe changed. If you have ever been at high elevation in the mountains where there is less oxygen in the air, you might have had a similar experience with difficulties breathing or headaches.
Coral reefs in the Red Sea
In addition to harming marine life, ocean acidification is hurting certain industries, creating economic stress. There is a decline in commercial fisheries, especially those that trade in lobster, scallops, and other shellfish. California, which is home to 31 different kinds of salmon and trout, is predicted to lose 23 of these species within the next century. More gravely, in many fishing villages in Indonesia, The Philippines, and Malaysia, fishing is necessary for survival. Hundreds of communities like these around the world must fish to feed themselves, so the depletion of their food source is a serious issue.
Fortunately, ocean acidification is a somewhat gradual process, giving us time to recognize the impact of human activity and change our behaviors to lessen harmful disruption to our oceans. To lower your own individual carbon dioxide emissions (or “carbon footprint”), you can use less electricity, recycle, and reducing use of your car by biking, walking, or using public transportation instead. If you want to find out your carbon footprint, you can visit The Nature Conservancy’s calculator here. More powerful ways to help the oceans are to support political measures that combat increasing carbon emissions and to donate to or volunteer with local organizations that champion environmental causes. One example is the Surfrider Foundation - an international organization that promotes the health of our oceans and beaches.
Fishermen in Pangadaran, Indonesia
Carbon dioxide is estimated to exist in the atmosphere for hundreds of years, so even if we cut off all carbon emissions today, we will not see a reversal of ocean acidification immediately. By teaching all of our friends and family about how we affect our environment, and by encouraging everyone to reduce our carbon footprints, we can thank our oceans for all that they give to us and ensure that our beautiful oceans and all of its organisms will exist in the future.
For another informative post about ocean acidification, check out John Hawthorne's article https://moboxmarine.com/blog/oceans-are-getting-acidic/
References
· Bland, Alistair. “Many Of California’s Salmon Populations Unlikely To Survive The Century.” The Salt. NPR, 17 May 2017. http://www.npr.org/sections/thesalt/2017/05/17/528826774/many-of-california-s-salmon-populations-unlikely-to-survive-the-century. 19 May 2017.
· Brewer, Peter G. and James Barry. “Rising Acidity in the Ocean: The Other CO2 Problem.” Sustainability. Scientific American, 1 September 2008. https://www.scientificamerican.com/article/rising-acidity-in-the-ocean/. 17 May 2017.
· “Ocean Acidification.” Pristine Seas. National Geographic. http://ocean.nationalgeographic.com/ocean/explore/pristine-seas/critical-issues-ocean-acidification/. 18 May 2017.
· “Ocean Acidification.” The Ocean Portal Team and Jennifer Bennett. Ocean Portal. Smithsonian National Museum of Natural History. http://ocean.si.edu/ocean-acidification. 18 May 2017.
· “Ocean Acidification.” Know Your Ocean. Woods Hole Oceanographic Institute. http://www.whoi.edu/ocean-acidification/. 16 May 2017.
Images:
All images used were found on Wikimedia Commons and are Public Domain